菅沼伸哉君・西田篤史君が中心となって行ったメタン変換触媒に関する九州大、産総研、北九州市立大との共同研究の成果がACS Catalysis誌 (ACS)にAcceptされました。Our collaboration with Kyusyu Univ., AIST, Kitakyusyuu City Univ. was published in ACS Catalysis (ACS).
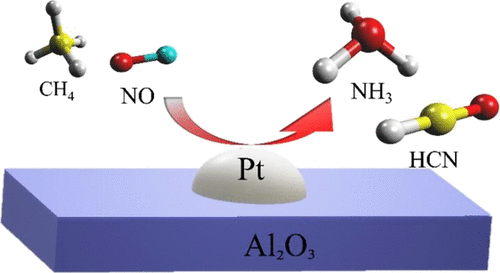
「Low-Temperature Activation of Methane with Nitric Oxide and Formation of Hydrogen Cyanide over an Alumina-Supported Platinum Catalyst」Tatsuya Yamasaki, Atsushi Nishida, Nobuya Suganuma, Yang Song, Xiaohong Li, Junichi Murakami, Tetsuya Kodaira, Kyoko K. Bando, Tatsumi Ishihara, Tetsuya Shishido, Atsushi Takagaki
ACS Catalysis, 2021, 11, 23, 14660-14668. DOI: 10.1021/acscatal.1c04548
This study demonstrated methane activation with subsequent conversion to hydrogen cyanide (HCN) at low temperatures using nitric oxide (NO) as the sole oxidant together with an alumina-supported platinum catalyst (Pt/Al2O3). This process afforded HCN even at 300 °C, indicating that C-H bond cleavage, NO dissociation, and simultaneous C-N coupling all occurred at this reduced reaction temperature. The HCN yield increased with increasing temperature, resulting in a 3.2% yield with a selectivity of 49% at 425 °C. This yield was much greater than that obtained from the reaction of CH4 with NH3 and O2, which suggests a reaction mechanism different from the Andrussow process. The HCN production rate was 11.4 mmol g-1 h-1 and the corresponding turnover frequency was 253 h-1, both of which were far superior to those obtained in previous studies at similar reaction temperatures. The Pt catalyst was found to be stable and could continuously produce HCN for at least 100 h. In situ X-ray absorption fine structure analyses suggested that the high resistance of this material to deep oxidation facilitated HCN formation. The difference between X-ray absorption near-edge structure spectra before and during the reaction indicated that the specific adsorbates on the catalyst were dependent on the reaction temperature and that the extent of adsorbed Pt-CN species was correlated with the reactivity of the material.