Our paper reporting hydrosilylation of internal alkynes by palladium-gold alloy catalyst was published in Organometallics (ACS).
[Featured on a supplementary journal cover on the journal's website]
「Importance of the Pd and Surrounding Sites in Hydrosilylation of Internal Alkynes by Palladium-Gold Alloy Catalyst」.
Sadhukhan, Tumpa; Junkaew, Anchalee; Zhao, Pei; Miura, Hiroki; Shishido, Tetsuya; Ehara, Masahiro
Organometallics, 2020 DOI:10.1021/acs.organomet.9b00745
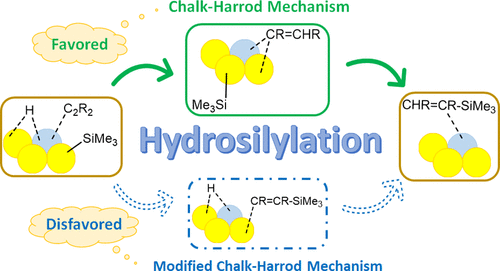
Recently, the Pd-Au alloy catalyst has been developed for the hydrosilylation of internal alkynes as well as α,β-unsaturated ketones under mild conditions. In this work, the mechanism of the hydrosilylation reaction of internal alkynes on the Pd-Au bimetallic cluster and the Pd-Au(111) alloy surface has been investigated by density functional theory calculations. The calculated energy profiles show that the reaction follows the Chalk-Harrod mechanism. The rate-determining step is the hydrometalation in the Pd-Au cluster while it is the Si-C reductive elimination in the Pd-Au(111) alloy surface. The Pd site acts as the adsorption site and the reactive center as observed in experiments. The surrounding Pd-Au bridge and Au sites are also relevant for the bond activation and accepting the substrates or intermediates during the reaction, which is characteristic in the Pd-Au alloy catalysts and not available in the homogeneous catalyst. The hydrosilylation reaction proceeds without too much high energy barriers and too stable intermediates in the Pd-Au alloy catalyst. The monometallic Au and Pd catalysts are not preferable because of the high energy barriers (Au) or too stable intermediates (Pd). The present picture of the relevance of the Pd atomic site and its surrounding Pd-Au bridge or Au sites will be useful for developing the alloy catalysts for the related catalytic reactions.