相原健司君が中心となって行ったPt/WO3/Al2O3触媒による水素化分解の反応機構に関する研究成果がCatalysis Today誌 (Elsevior)にAcceptされました。Our paper reporting the mechanism of the selective hydrogenolysis of C-O bonds over a Pt/WO3/Al2O3 catalyst was accepted in Catalysis Today (Elsevior).
「Investigation of the mechanism of the selective hydrogenolysis of C-O bonds over a Pt/WO3/Al2O3 catalyst」
Aihara, Takeshi; Miura, Hiroki; Shishido, Tetsuya
Catalysis Today, 2020, 352, 73-79. DOI:10.1016/j.cattod.2019.10.008
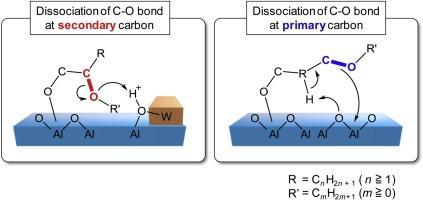
The hydrogenolysis of various polyols and ethers over a Pt/WO3/Al2O3catalyst was investigated. The hydrogenolysis rate of a C-O bond at a secondary carbon was higher than that at a primary carbon, indicating that a hydroxyl (OH) group at a primary carbon played an important role in the dissociation of a C-O bond. Moreover, the dissociation position of the C-O bond in alcohols and ethers strongly depended on the stability of the carbocation intermediate, and hydrogenolysis via a secondary carbocation as an intermediate proceeded preferentially to that via a primary carbocation. The kinetics of the hydrogenolysis of C3 polyols were also investigated. The reaction orders with respect to the concentrations of glycerol, 1,2- and 1,3-propanediol were almost the same. In contrast, different reaction orders with respect to the H2pressure were observed for the hydrogenolysis of C3 polyols with or without vicinal OH groups, indicating that the dissociation of a C-O bond at primary and secondary carbons proceeded via completely different mechanisms. These investigations suggested that both the structure and position of a substrate on the catalyst surface must be controlled for highly selective hydrogenolysis.