Our paper reporting selective reduction of NO by Rh/NbOPO4 catalyst was accepted in Catalysis Today (Elsevier).
「Selective catalytic reduction of NO with CO and C3H6 over Rh/NbOPO4」.
Imai, Shinsuke; Miura, Hiroki; Shishido, Tetsuya
Catalysis Today, 2019, 332, 267-271 DOI:10.1016/j.cattod.2018.07.027
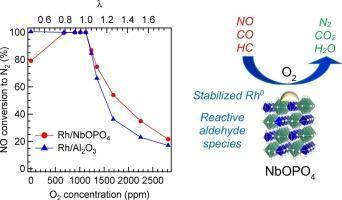
The selective catalytic reduction of NO with C3H6 and CO was investigated over platinum group metal (PGM; Pd, Rh, or Pt) supported on NbOPO4. Rh/NbOPO4 exhibits a lower light-off temperature of NO reduction to N2 compared to Pd- or Pt-loaded NbOPO4 catalysts and Rh/Al2O3 under stoichiometric conditions. Rh/NbOPO4 shows superior catalytic activity for NO reduction to Rh/Al2O3 under a wide range of lean conditions. Propylene (C3H6) was partially oxidized to reactive aldehyde species on Rh/NbOPO4, whereas less-reactive carboxylate species was generated and adsorbed on Rh/Al2O3. A highly dispersed Rh0nanoparticles on NbOPO4 was slowly oxidized by exposure to excess O2, but Rh on Al2O3was readily oxidized to less-active Rh2O3. The high activity of Rh/NbOPO4 for C3H6 oxidation to reactive aldehyde species and the resistance of Rh0 against oxidation results in the enhancement of NO reduction activity in a slightly lean region.