Our paper reporting dehydrogenation of ethylbenznene catalyzed by supported Pt-Sn alloy catalysts was published in Industrial & Engineering Chemistry Research (ACS).
「Highly active and stable Pt-Sn/SBA-15 catalyst prepared by direct reduction for ethylbenzene dehydrogenation: Effects of Sn addition」DENG, Lidan; Arakawa, Takuto; Ohkubo, Tomoyo; Miura, Hiroki; Shishido, Tetsuya; Hosokawa, Saburo; Teramura, Kentaro; Tanaka, Tsunehiro
Industrial & Engineering Chemistry Research, 2017, DOI: 10.1021/acs.iecr.7b01598
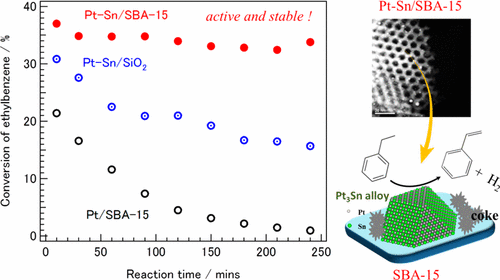
A series of Pt-Sn/SBA-15 catalysts (Sn/Pt nominal ratios: 0-3) prepared by direct reduction were applied to ethylbenzene dehydrogenation to styrene. The characterization by X-ray diffraction, X-ray absorption fine structure, CO adsorption, transmission electron microscopy and X-ray photoelectron spectroscopy revealed the formation of highly dispersed and stable Pt-Sn alloy particles (PtxSny (x/y≧3) and PtSn alloys having Sn-rich surfaces) on SBA-15. Non-alloyed Sn existed as highly dispersed SnO2. 1Pt1Sn/SBA-15 (Sn/Pt nominal ratio=1) exhibited the highest activity, on which Pt3Sn alloy nanoparticles were mainly formed. On the other hand, PtSn alloy was dominant on Pt-Sn/SBA-15 catalysts whose Sn/Pt nominal ratios were larger than 1, and the activity was decreased. Furthermore, 1Pt1Sn/SBA-15 exhibited a higher stability than Pt/SBA-15 and 1Pt1Sn/SiO2. The addition of Sn not only inhibits C-C bond cleavage, improves selectivity towards styrene, but also enhances the "drain-off" effect--allowing coke precursors migrate from the active metals to SBA-15 with large surface area.