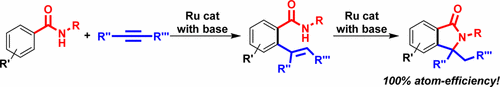
Our paper reporting atom-economical synthesis of Isoindolinones by Ru catalysts was published in The Journal of Organic Chemistry (ACS).
「Ruthenium-Catalyzed Addition of Aromatic Amides to Internal Alkynes and Subsequent Intramolecular Cyclization for the Atom-Economical Synthesis of Isoindolinones」Hiroki Miura, Sachie Terajima, Kentaro, Tsutsui, Tetsuya Shishido
The Journal of Organic Chemistry, 2017, 82, 1231-1239 DOI: 10.1021/acs.joc.6b02552
A selective and atom-economical synthesis of isoindolinones is described. This novel synthetic strategy involves two catalytic reactions: the ruthenium-catalyzed regioselective alkenylation of aromatic C-H bond of aromatic amides with internal alkynes, and subsequent intramolecular cyclization of the resulting alkene with amide functionalities. The addition of only a catalytic amount of bases is required for efficient construction of the desired isoindolinones, and no byproducts are formed in the tandem catalytic reactions. Various kinds of aromatic amides and internal alkynes can be used in the present reactions, and the corresponding isoindolinones bearing a quaternary carbon at the C3 position are obtained in good to high yields.